Steripath®, proven clinical and cost effectiveness in 20+ studies
All reporting ≤ 1% sustained contamination rate.
University of Nebraska Medical Center
Reduction in Blood Culture Contamination Through the Use of Initial Specimen Diversion Device® [Steripath®]
- Publication: Clinical Infectious Diseases (2017)
- Institute: University of Nebraska Medical Center
- Authors: Mark E. Rupp, MD, et al
- Affiliations: Division of Infectious Disease, Department of Epidemiology, Emergency Department
- Design: Single-center, prospective, controlled, matched-pair, open-label trial over a 12-month period – 904 patients (1,808 cultures)
- Method: Phlebotomists collected two cultures from each subject. 1) One using Phlebotomy best practices. 2) One using Steripath
- Results: Use of Steripath resulted in a 92% reduction in contamination rate compared to pre-intervention rate of 2.6%. 88% reduction during the 12-month study period, ISDD: 0.2% vs. standard method: 1.8%. Sensitivity was not compromised, and researchers calculated that the study institution would save $1.8M/year with Steripath
Lee Health
Effectiveness of a Novel Blood Culture Collection System in Reducing Blood Culture Contamination Rates in the ED
- Publication: Journal of Emergency Nursing (2018)
- Institute: Lee Health (multi-center trial n=4)
- Authors: Mary Bell, MSN, RN, CEN, et al
- Affiliations: Department of Emergency Medicine
- Method: Blood cultures contamination rates with Steripath collected via peripheral IV start and venipuncture were compared with historical rates via standard method.
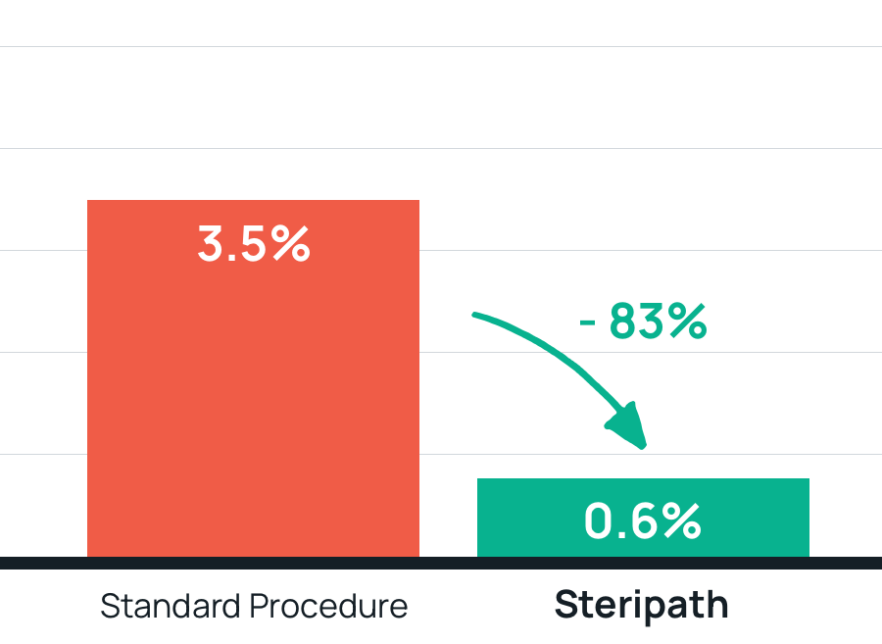
- Results: 83% reduction in contamination with Steripath
Steripath: 0.6% (38/6,293) contamination rate (P=0.0001)
Standard procedure: 3.5% (1,246/35,392) contaminate rate - Summary: Prevented 184 false-positive events.
86% of Steripath draws are via PIV starts.
Cost savings of $641,792 during a 7-month trial period.
U.S. Department of Veterans Affairs
Asynchronous Testing of 2 Specimen Diversion Devices to Reduce Blood Culture Contamination: A Single-Site Product Supply Quality Improvement Initiative
- Publication: Journal of Emergency Nursing (2021)
- Institute: Central Texas Veterans Health Care System
- Authors: Monica Arenas, MS, et al
- Affiliations: Adult Emergency Department
- Method: Blood cultures were obtained over a 5-month period using Steripath (Device A arm of study) via peripheral IV start or venipuncture. Cultures drawn without a device were used to compare rates. Steripath was not used in the Device B arm of study.
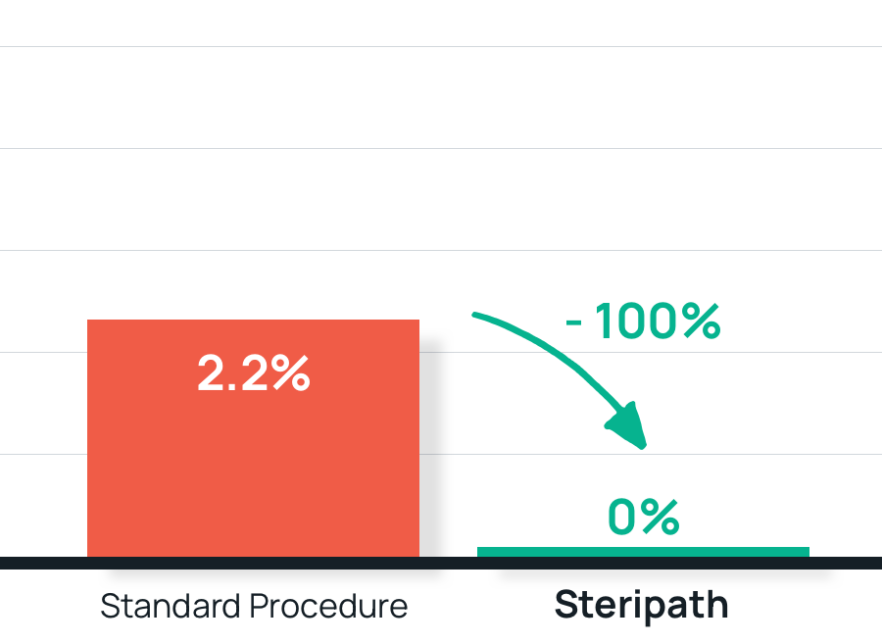
- Results: 100% reduction in contamination with Steripath
Steripath: 0.0% (0/664) contamination rate
Standard Method: 2.2% (17/761) contaminate rate - Summary: Recommendation to use ISD devices as a QI bundle along with best practice site preparation and phlebotomy.
Brooke Army Medical Center (BAMC)
Initial Specimen Diversion Device® Reduces Blood Culture Contamination and Vancomycin Use in Academic Medical Center
- Publication: Journal of Hospital Infection (2021)
- Institute: Brooke Army Medical Center
- Authors: Lindsey Nielsen, PhD, ASCP(M,MB), et al
- Affiliations: Pathology, Lab Services, Emergency Medicine, and Infectious Disease
- Design: Single-center, prospective, open-label trial
- Method: Blood cultures were collected in the Emergency Department. Patients were randomized to either standard method or use of Steripath via peripheral IV starts and venipuncture.
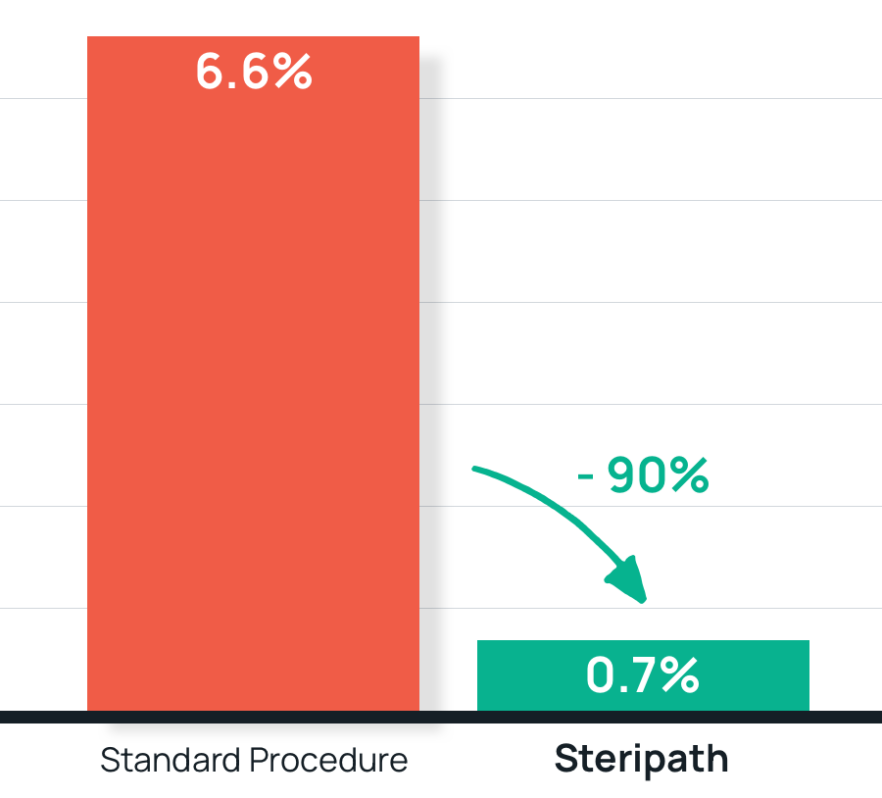
- Results: 90% reduction in contamination with Steripath
Steripath: 0.7% (7/1,016) contamination rate (P=0.0001)
Standard procedure: 6.6% (53/800) contamination rate - Summary: Steripath was adopted as standard practice hospital-wide for eligible, (non-pediatric) patients.
Brooke Army Medical Center (BAMC)
Initial Specimen Diversion Device® Reduces Blood Culture Contamination and Vancomycin Use in Academic Medical Center
- Publication: Journal of Hospital Infection (2021)
- Institute: Brooke Army Medical Center
- Authors: Lindsey Nielsen, PhD, ASCP(M,MB), et al
- Affiliations: Pathology, Lab Services, Emergency Medicine, and Infectious Disease
- Design: Single-center, retrospective, non-randomized
- Method: Comparison of Vancomycin DOT before/after interventions to reduce pathogen detection time (NAAT) and blood culture contamination (Steripath®) in the ED. Hospital-wide vancomycin DOT collected through EMR.
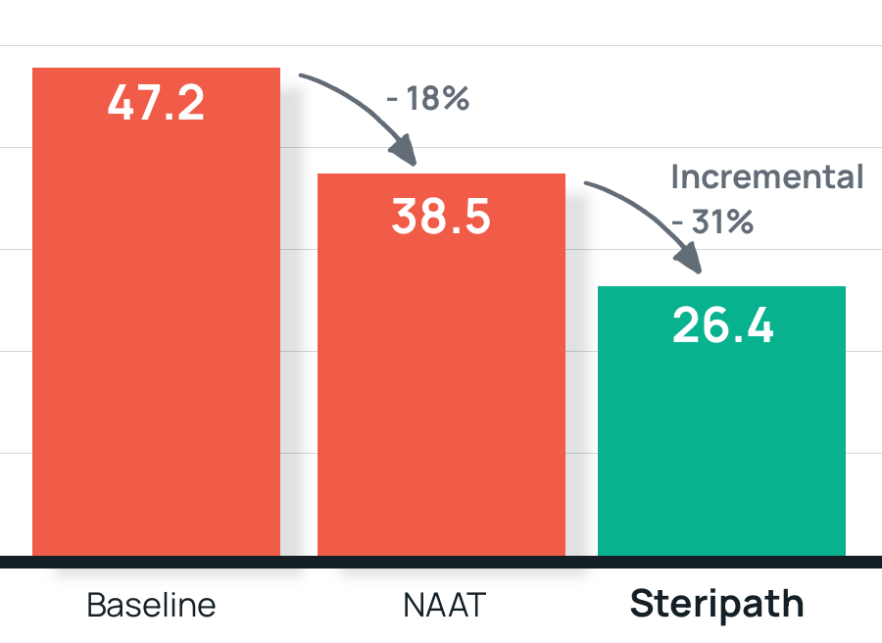
- Results: Vancomycin DOT per 1,000 patient days decreased 18% (47.2 +/-5.4 to 38.5 +/-13.3) after implementation of NAAT.
Steripath resulted in a significant incremental decrease in vancomycin DOT by 31% (38.5 +/-13.3 to 26.4 +/- 6.2) - Summary: Blood culture contamination rate was not significantly altered after implementation of rapid molecular PCR identification method. Reducing contamination with Steripath contributed to a significant reduction in unnecessary antibiotic therapy.
Stanford Health Care
Getting to Zero: Impact of a Device (Steripath®) to Reduce Blood Culture Contamination and False-Positive Central Line-Associated Bloodstream Infections
- Publication: Infection Control & Hospital Epidemiology (2022)
- Institute: Stanford Hospital and Clinics
- Authors: Lucy Tompkins, MD, PhD, et al
- Design: Single-center, prospective, controlled study March 2019–January 2020 (10-months)
- Method: Blood cultures were obtained hospital-wide by Phlebotomy team using the Steripath Gen2 compared to standard method.
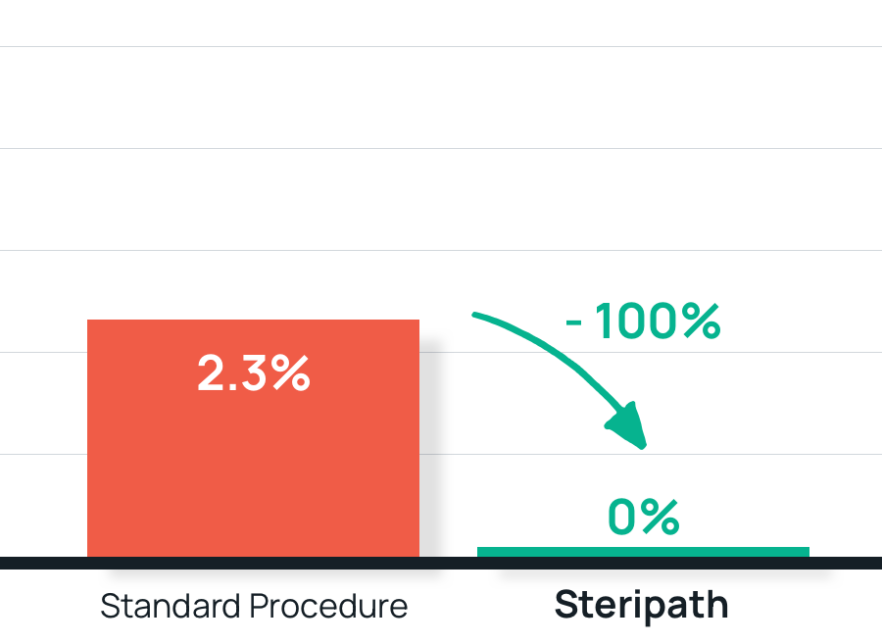
- Results: 100% reduction in blood culture contamination Steripath Gen2: 0.0% (0/11,202) contamination rate Standard method: 2.3% (111/4,759) contamination rate 12-Fold decrease in NHSN/CMS reportable False-Positive CLABSIs
- Steripath Gen2: 1
- Standard method: 12
- SIR fell by 30-50% when contaminants were removed
WVU Medicine – United Hospital Center
Initial Specimen Diversion Device Utilization Mitigates Blood Culture Contamination Across Regional Community Hospital and Acute Care Facility
- Publication: American Journal of Medical Quality (2022)
- Institute: WVU Medicine – United Hospital Center
- Authors: Mark Povroznik, MD
- Design: Hospital-wide, single-center, prospective, controlled study over an 8-month period
- Method: Emergency Department staff, laboratory phlebotomists, and nursing staff on acute-critical care floors used Steripath ISDD to collect blood cultures via peripheral IV start or venipuncture.
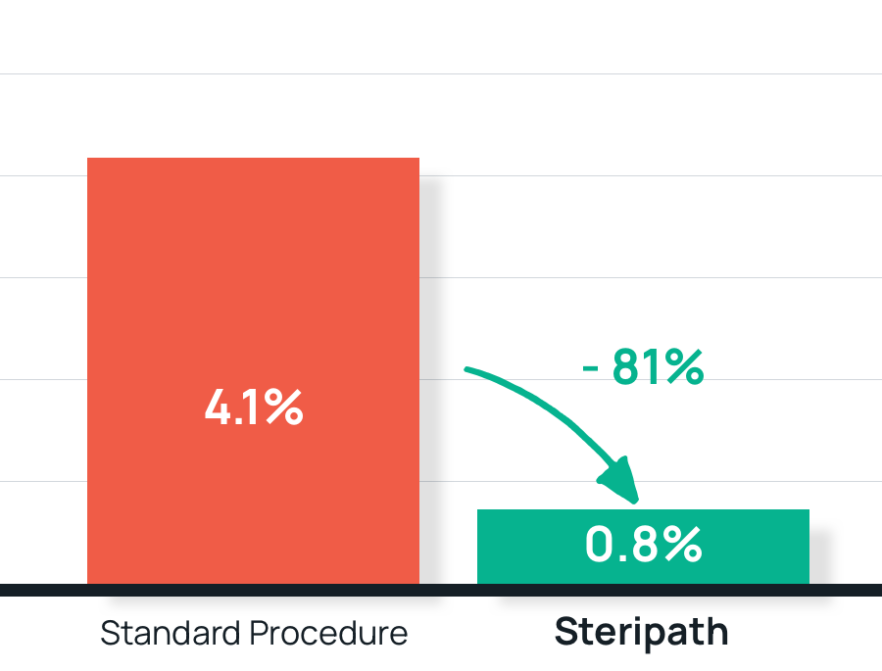
- Results: 81% reduction in contamination with Steripath
Steripath: 0.8% (36/4,631) rate (P<0.00001) – 82% compliance
Standard method: 4.1% (41/1,011) contamination rate - Summary: Steripath was adopted as standard practice for hospital-wide blood culture collection.
Shaare Zedek Medical Center
Reducing Blood Culture Contamination Using the Initial Specimen Diversion Device
- Publication: American Journal of Infection Control (2019)
- Institute: Shaare Zedek Medical Center
- Authors: Frederic S. Zimmerman, MD, et al
- Affiliations: Department of Intensive Care, Laboratory of Clinical Microbiology, Department of Internal Medicine, Infectious Disease Unit
- Design: Single-center, prospective, controlled pragmatic study
- Method: Blood cultures were obtained using the Initial Specimen Diversion Device, either via integrated needle or attachment to a newly placed intravenous catheter. Cultures taken using standard methods served as the control (n=671 blood cultures).
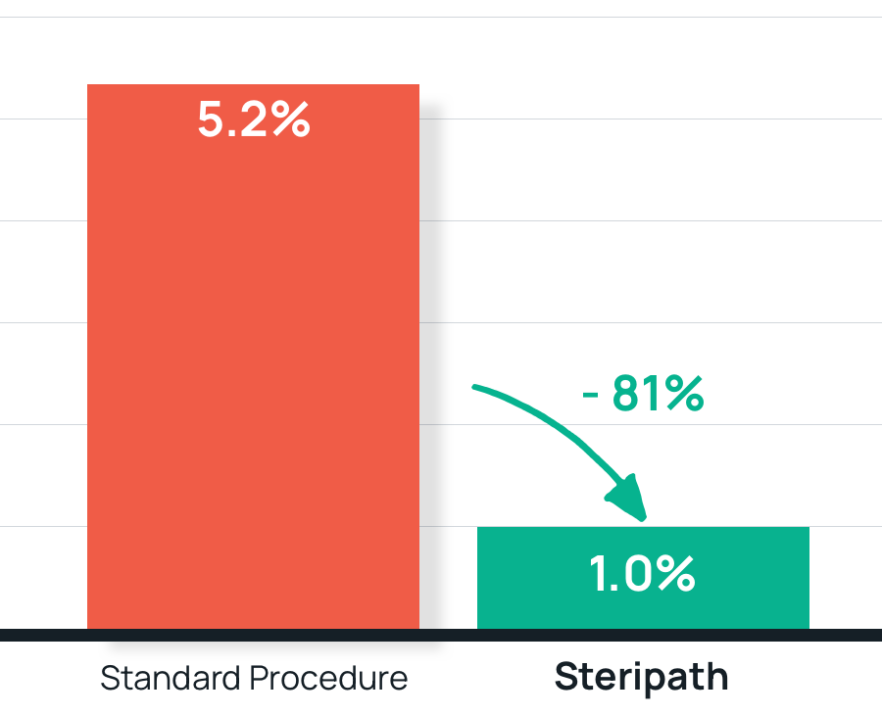
- Results: 81% reduction in blood culture contamination using Steripath
Steripath: 1.0% contamination rate (P=0.008)
Standard procedure: 5.2% contamination rate - Summary: The use of the ISDD was associated with reduced culture contamination in hospitalized patients over a 6-month period, without concomitant reduction in true-positive cultures.
Inova Health System
Don’t Stick Me Again – Reducing Blood Culture Contamination in the Adult Emergency Department
- Conference: ENA Conference Award Winner (2019)
- Institute: Inova Fairfax Hospital
- Authors: Kara Bauman, MN, RN, CEN, CPEN, TCRN
- Affiliations: Adult Emergency Department
- Design: Single-center, prospective, controlled, non-randomized trial over a 12-month period
- Method:The Steripath ISDD was used for blood culture collection via peripheral IV starts and venipuncture. Standard method (SM) was used to collect cultures from some patients with difficult vein access or other factors.
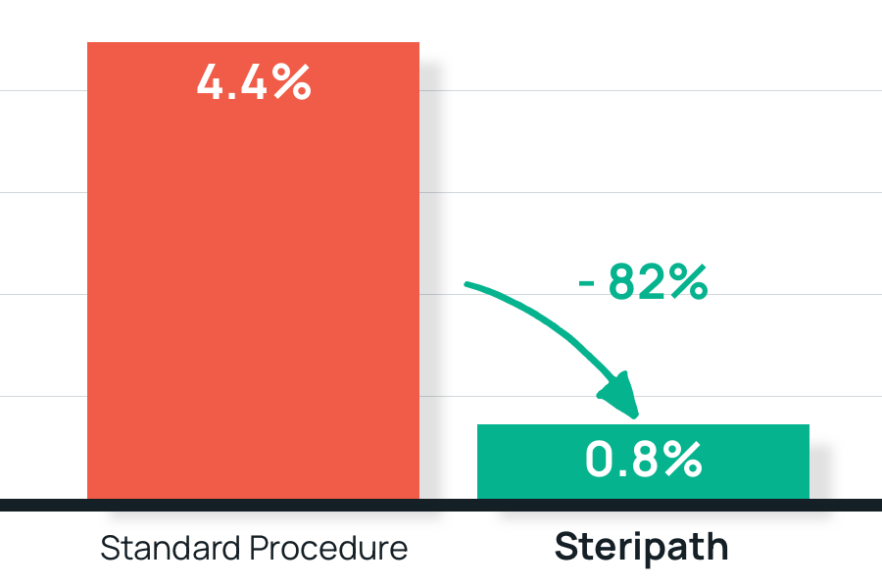
- Results: 82% reduction in blood culture contamination Steripath: 0.8% (90/10,879) contamination rate – 80% compliance Standard procedure: 4.4% (121/2,720) contamination rate
- Summary: Reduced costs. Promoted antibiotic stewardship. 69% of Steripath draws were via PIV starts.
Baylor Scott & White
Emergency Department Blood Culture Contamination Rate Substantially Reduced with Initial Specimen Diversion Device
- Conference: ENA (2021)
- Institute: Baylor Scott & White Centennial Medical Center
- Authors: Carleen Merola, DNP, RN, TCRN, PCCN
- Affiliations: Emergency Department
- Design: Prospective, non-randomized cohort study over a 4-month period
- Method: Blood cultures were obtained using the initial specimen diversion device, via peripheral IV start and venipuncture. Cultures taken using standard methods served as control.
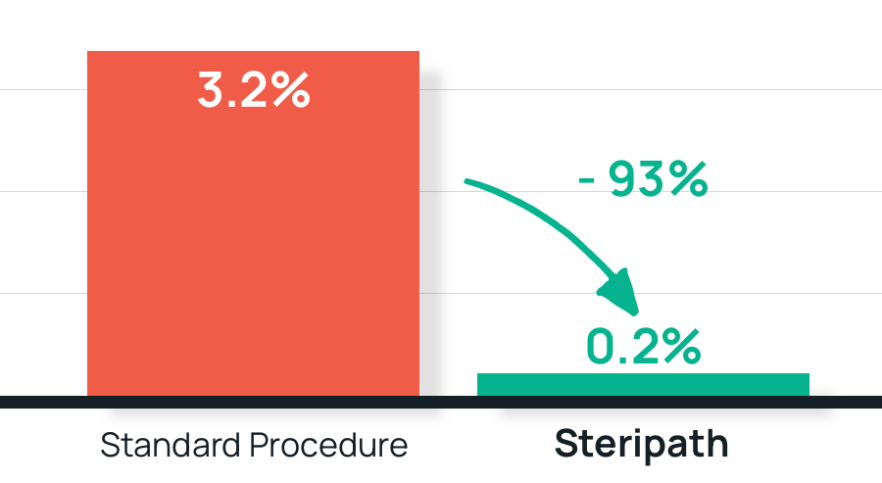
- Results: 93% reduction in blood culture contamination
Steripath: 0.2% (1/448) contamination rate (P = 0.0002%)
Standard procedure: 3.2% (17/527) contamination rate - Summary: Based on the observed results, Steripath was adopted as standard practice hospital-wide and they strongly advocate consideration of this technology by nursing leaders and quality control personnel as a means of improving patient outcomes.
Kern Medical
Emergency Department Blood Culture Contamination Rate Substantially Reduced with Initial Specimen Diversion Device
- Conference: APIC (2021) (submitted for publication)
- Institute: Kern Medical Center
- Authors: Kristi Brownfield, MSN, RN, CIC, et al
- Affiliations: Emergency Department, Clinical Microbiology and Infection Prevention, and Employee Health
- Design: Single-center, prospective, controlled study over an 18-month period
- Method: Blood cultures were obtained using the initial specimen diversion device, via peripheral IV start and venipuncture. Cultures taken using standard methods served as control.
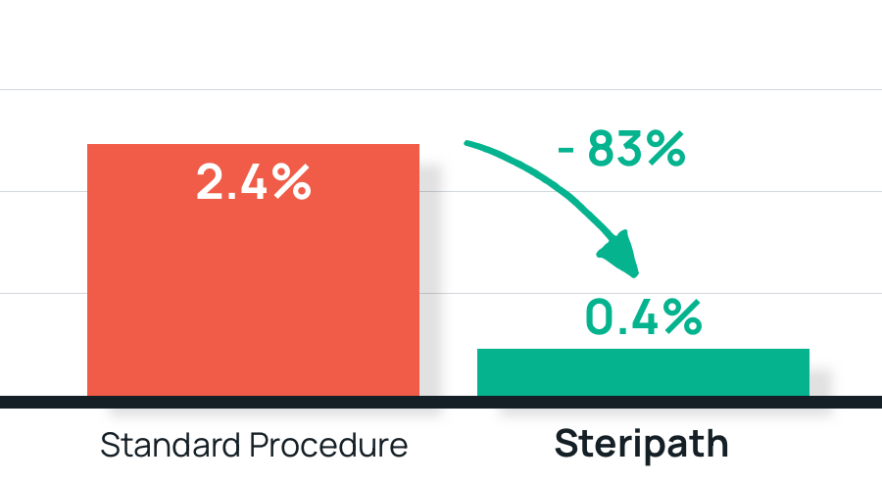
- Results: 83% reduction in blood culture contamination using Steripath
Steripath: 0.4% (9/2,168) contamination rate
Standard procedure: 2.4% (77/3,170) contamination rate - Summary: The use of the Steripath ISDD was associated with a significant sustained reduction in blood culture contamination in the ED and supported the adoption of ISDD as standard practice.
Ascension Via Christi
Antimicrobial Stewardship Standards and Patient Safety: A Case Study in Blood Culture Contamination
- Conference: SHEA (2021)
- Institute: Ascension Via Christi Health
- Authors: Connie L. Schaefer, RN, CIC
- Affiliations: Emergency Departments and Infection Prevention at Via Christi Health (multi-center trial n=3)
- Design: Multi-center, prospective, non-randomized cohort study over a 3-month period
- Method: Blood cultures were obtained using the initial specimen diversion device, either via peripheral IV start or venipuncture. Cultures taken using standard methods served as control.
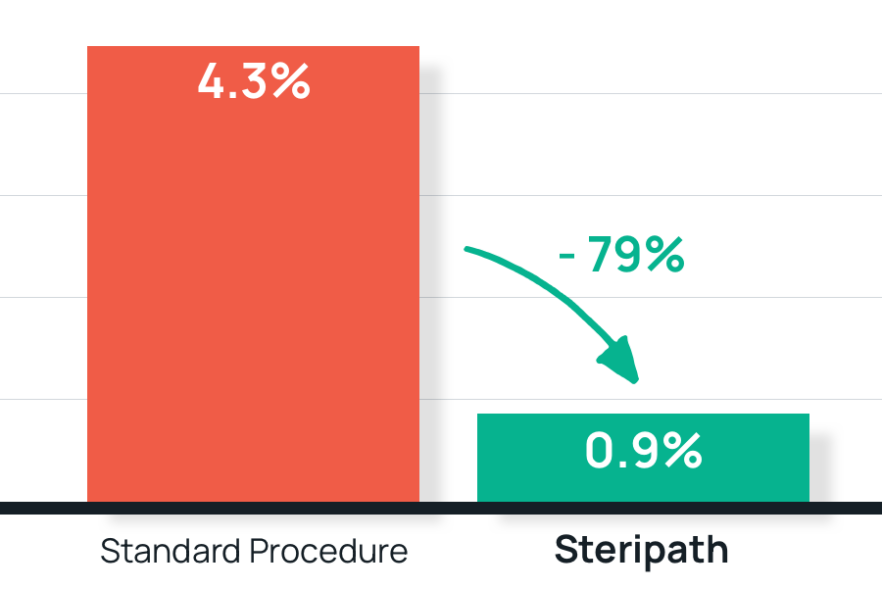
- Results: 79% reduction in blood culture contamination
Steripath: 0.9% (16/1,847) contamination rate
Standard procedure: 4.3% (37/862) contamination rate - Summary: Implementation of the Steripath ISDD directly impacted patient outcomes and conserved laboratory and therapeutic resources.
SCL Health – St. Mary’s Medical Center
Reducing Blood Culture Contamination: New Best Practice Tool
- Conference: AONL (2020)
- Institute: SCL St. Mary’s Medical Center
- Authors: Grace Poteet, MSN, RN-BC, CEN, CPEN, TCRN Cindy Parrish, MT(ASCP), SC, SH, DLM
- Affiliations: Adult Emergency Department and In-patient
- Design: Single-center, prospective, controlled, non-randomized trial over a 6-month period
- Method: Steripath ISDD was used for blood culture collection via peripheral IV starts and venipuncture compared to historical standard method.
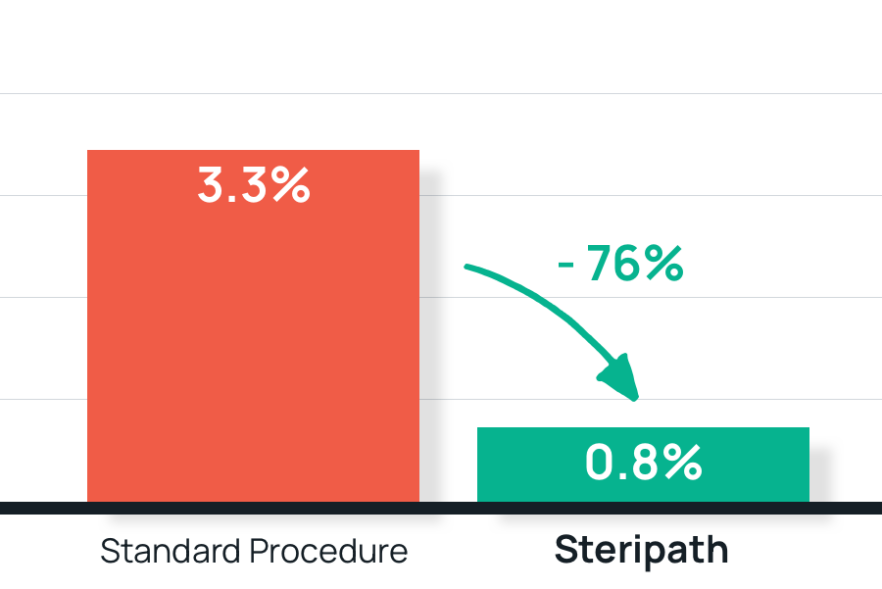
- Results: 76% reduction in blood culture contamination using Steripath
Steripath: 0.8% contamination rate
Standard method: 3.3% contamination rate - Summary: Success led to hospital-wide and system-wide Steripath adoption as standard practice. System-wide BCC rate has been sustained <1.0%.
Medical University of South Carolina
Reducing False Positive Blood Cultures in Adult Emergency Department
- Conference: Institute for Healthcare Improvement (2016)
- Institute: Medical University of South Carolina
- Authors: Lori Gauld, MT, SM, et al
- Affiliations: Department of Pathology and Laboratory Medicine, Adult Emergency Department
- Design: Single-center, prospective, open-label trial over an 8-month period
- Method: ED-dedicated nursing personnel were trained to use the Steripath device for blood culture draws from peripheral IV start or venipuncture. Floating nursing personnel were not trained on Steripath and utilized standard blood collection procedures and served as internal control.
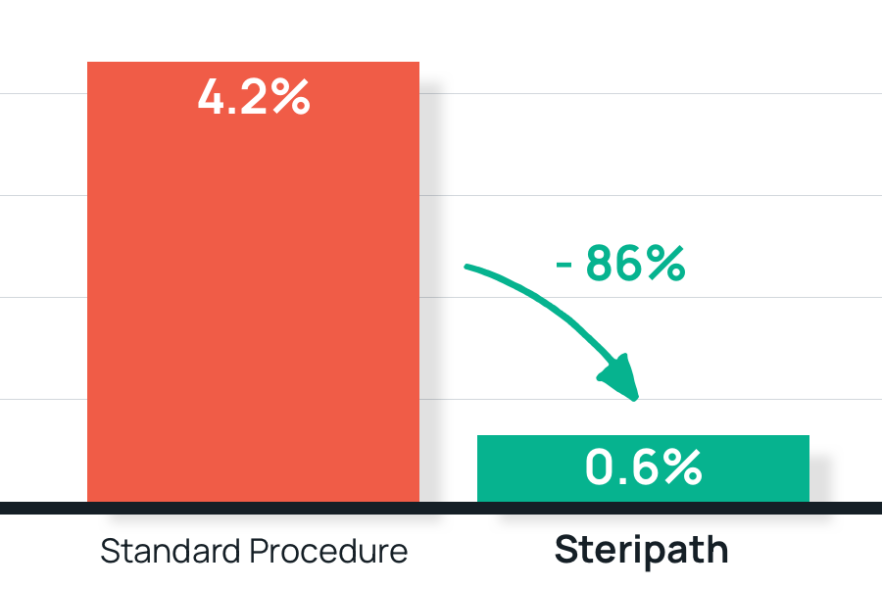
- Results: 86% reduction in blood culture contamination with Steripath
Steripath: 0.6% contamination rate
Standard procedure: 4.2% contamination rate - Summary: Steripath was adopted in the adult ED and was expanded to a second, separate adult ED and the ICU.
Beebe Healthcare
Reduction of Blood Culture Contamination Using Initial Specimen Diversion Device®
- Conference: ASM Conference (2018)
- Institute: Beebe Healthcare
- Authors: Jennifer Blakeney, MT(ASCP)SM
- Affiliations: Laboratory, Adult Emergency Department, and ICU
- Design: Single-center, prospective, non-randomized trial over a 4-month period
- Method: Blood cultures were collected hospital-wide. Patients were non-randomized to either standard procedure or Steripath for blood culture collection.
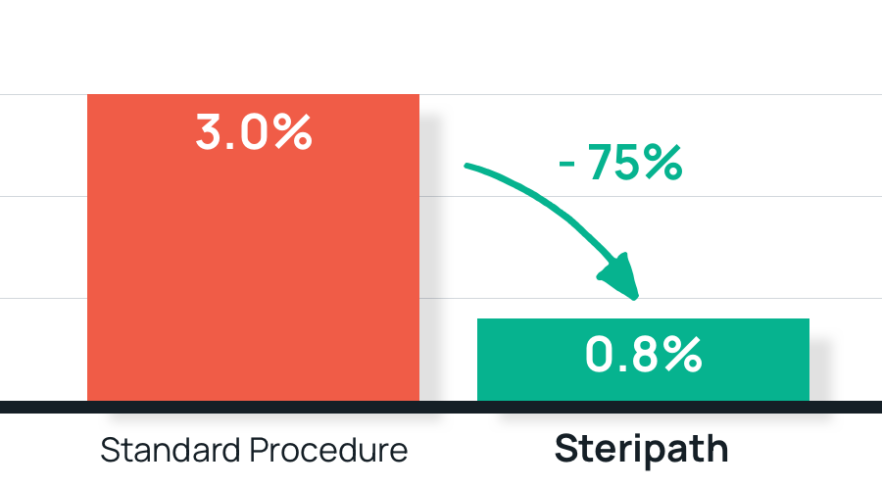
- Results: 75% reduction in blood culture contamination
Steripath: 0.8% (14/1837) contamination rate
Standard method: 3.0% (24/802) contamination rate - Summary: Hospital-wide adoption of Steripath as standard practice.
Rush University Medical Center
Significant Reduction of Blood Culture Contamination in the Emergency Department (ED) Using the Steripath® Blood Diversion Device
- Conference: IDWeek Conference (2017)
- Institute: Rush University Medical Center
- Authors: Chawat Tongma, MD, et al
- Affiliations: Emergency Department, Department of Pathology
- Design: Single-center, prospective, open-label trial
- Method: Pre-intervention blood cultures were collected using standard aseptic technique by nurses in the ED. During intervention, blood cultures were collected using the Steripath.
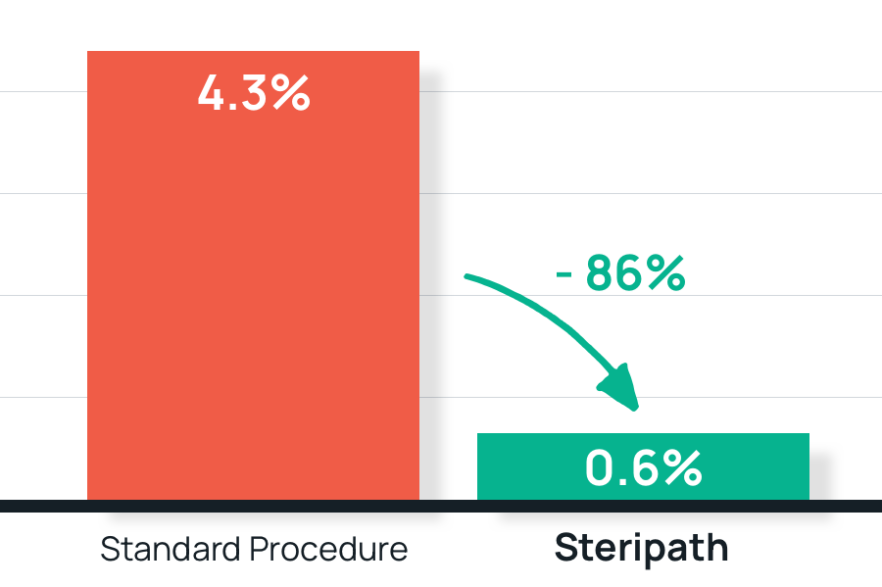
- Results: 86% reduction in blood culture contamination using Steripath
Steripath: 0.6% contamination rate
Standard procedure: 4.3% contamination rate - Summary: The Steripath device represents a simple and effective method for reducing blood culture contamination.
U.S. Department of Veterans Affairs
ER Pilot Leads to Hospital-Wide Implementation of Steripath® Initial Specimen Diversion Device®
- Conference: ENA Conference (2018)
- Institute: Michael E. DeBakey VA Medical Center, Houston
- Authors: Karen Stonecypher, PhD, MSN, RN, et al
- Affiliations: Emergency Department, ER Quality Improvement Committee
- Design: Single-center, prospective, non-randomized trial over a 2-month period
- Method: Blood cultures were collected in the Emergency Department. Patients were non-randomized to either usual care or Steripath for blood culture collection via peripheral IV starts and venipuncture.
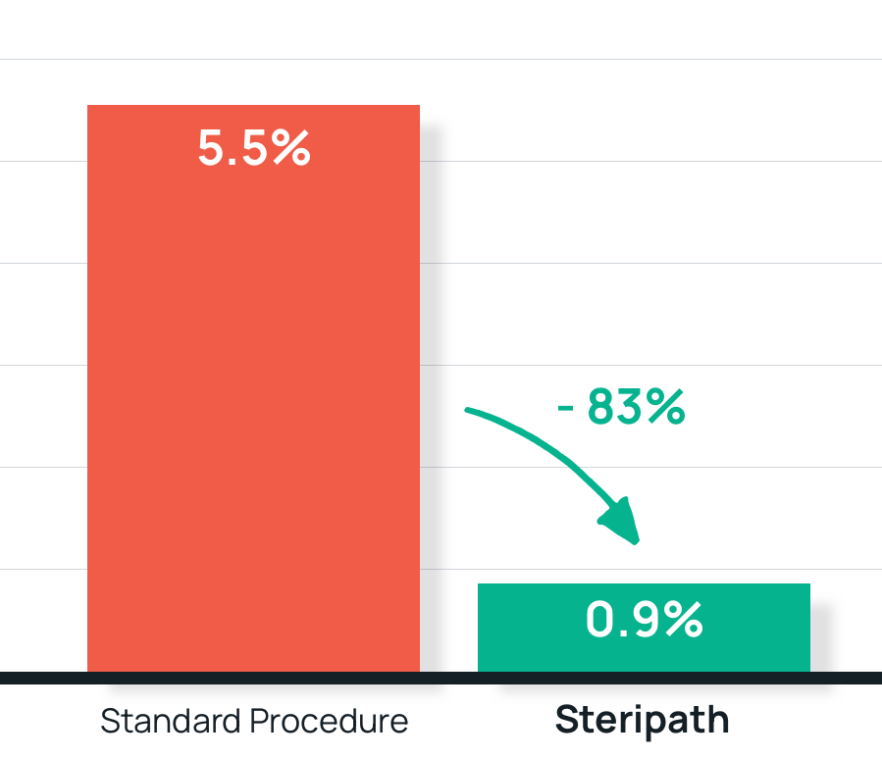
- Results: 83% reduction in blood culture contamination.
Steripath: 0.9% (3/321) contamination rate
Standard procedure: 5.5% (13/237) contamination rate - Summary: Hospital-wide adoption of Steripath as standard practice.
Medical University of South Carolina
Novel Blood Culture Collection Device Reduces False-Positive Blood Cultures, Saves Costs, and Increases Accuracy of Bloodstream Infection Diagnosis
- Conference: Institute for Healthcare Improvement National Forum (2017)
- Institute: Medical University of South Carolina
- Authors: Lisa L. Steed, PhD, D(ABMM), et al
- Affiliations: Department of Pathology & Laboratory Medicine, Adult Emergency Department
- Design: Single-center, prospective, open label trial
- Method: ED nurses were trained to collect blood cultures with Steripath® (ISDD®) via peripheral IV start or venipuncture.
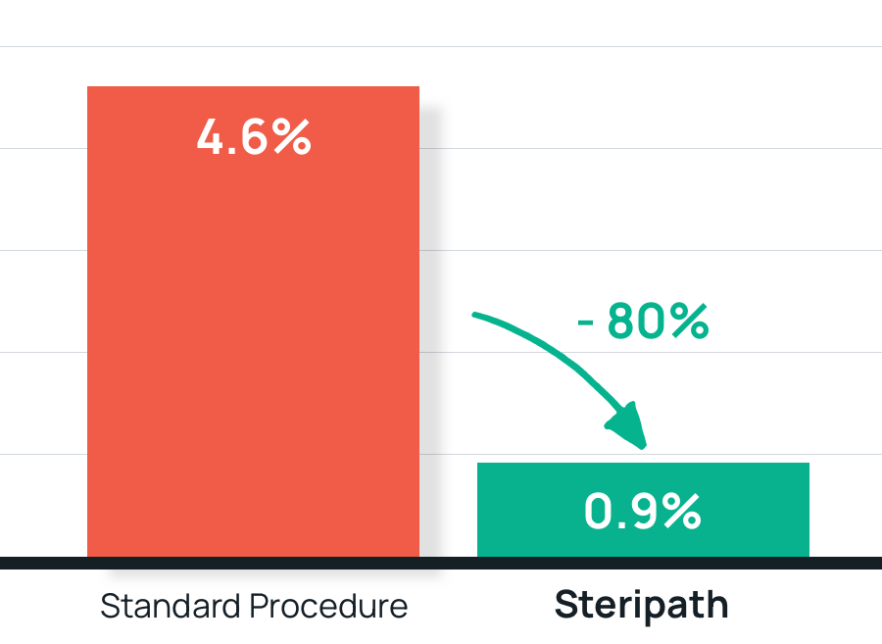
Trained ED nurses used their judgement on when to use the Initial Specimen Diversion Device®. - Results: 81% reduction in blood culture contamination;
Steripath: 0.9% rate;
Standard procedure: 4.6% rate. - Summary: Steripath (ISDD) use decreased false-positive blood cultures below 1%, in a busy adult ED. This reduction was sustained for 20 months.
University of Houston (College of Pharmacy)
Estimated Clinical and Economic Impact through Use of a Novel Blood Collection Device to Reduce Blood Culture Contamination in the Emergency Department: a Cost-Benefit Analysis
- Publication: Journal of Clinical Microbiology (2019)
- Institute: University of Houston (College of Pharmacy)
- Authors: Erik Skoglund, et al
- Affiliations: Pharmacy Practice and Translational Research, Pharmaceutical Health Outcomes and Policy, College of Pharmacy
- Design: Decision tree analysis model
- Method: Decision tree health care economic model to assess the cost benefit of routine use of Steripath® (ISDD®) in a health system ED and to evaluate the downstream clinical and economic impacts of routine use of Steripath.
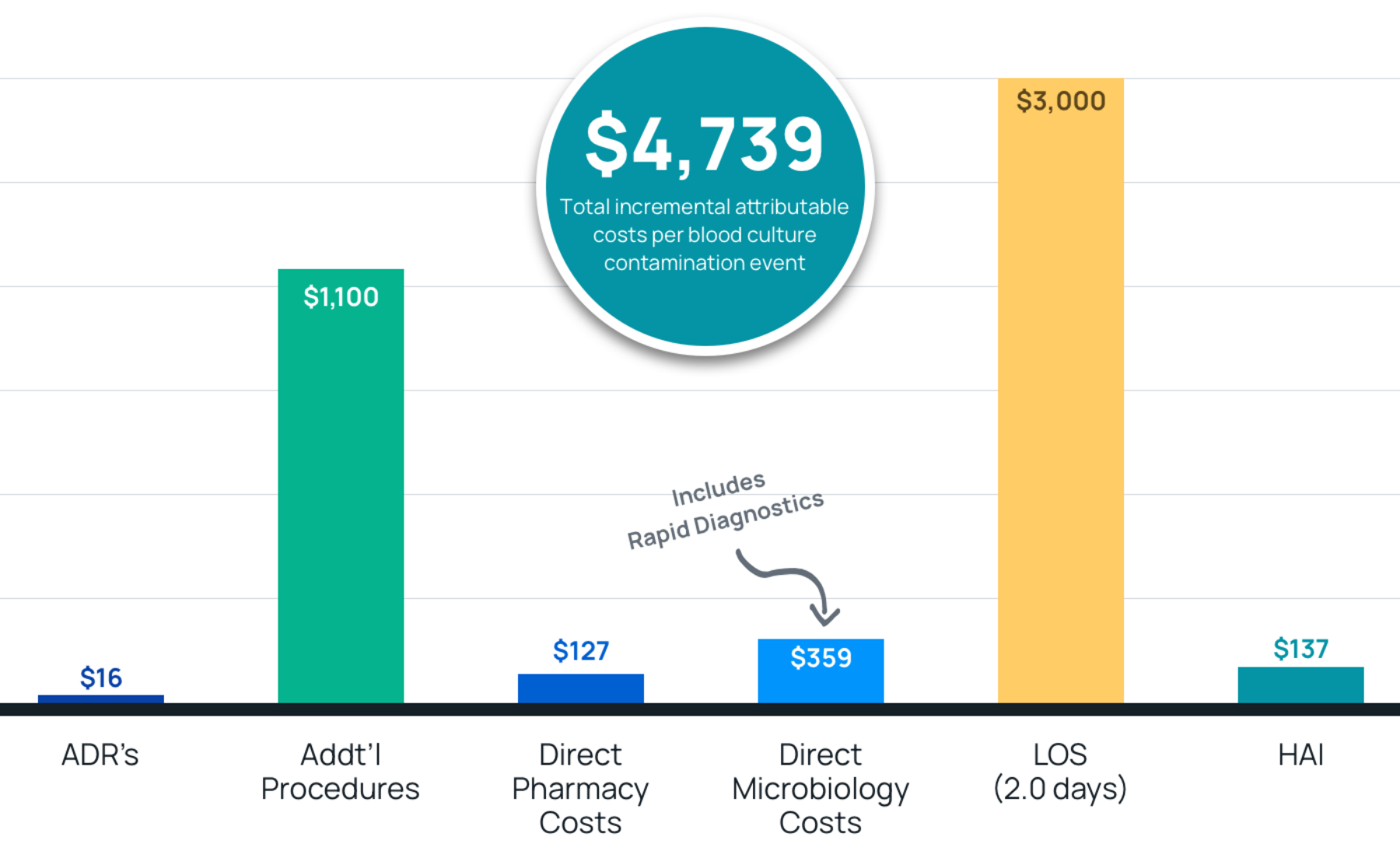
- Results: Total costs of $4,739 per contamination event with a length of stay increase of 2.0 days. Estimated costs savings were $79-367 per blood culture after adoption of Steripath.
- Summary: These findings support the routine use of Steripath for the collection of blood cultures in the ED as a cost-beneficial strategy to reduce the clinical and economic effect of blood culture contamination.
Massachusetts General Hospital, Harvard Medical School, Wing Tech Inc.
Model to Evaluate the Impact of Hospital-Based Interventions Targeting False-Positive Blood Cultures on Economic and Clinical Outcomes
- Publication: Journal of Hospital Infection (2019)
- Researchers: Massachusetts General Hospital, Harvard Medical School, Wing Tech Inc.
- Authors: B.P. Geisler, et al
- Affiliations: General Medicine and Infectious Diseases, Mass General Infectious Diseases, Harvard Medical School
- Design: Retrospective matched survival analysis
- Method: Based on hospitalized patients with septicemia-compatible symptoms. BCC costs, HACs, and potential savings were calculated based on the primary LOS data, a modified Delphi process, and published sources.
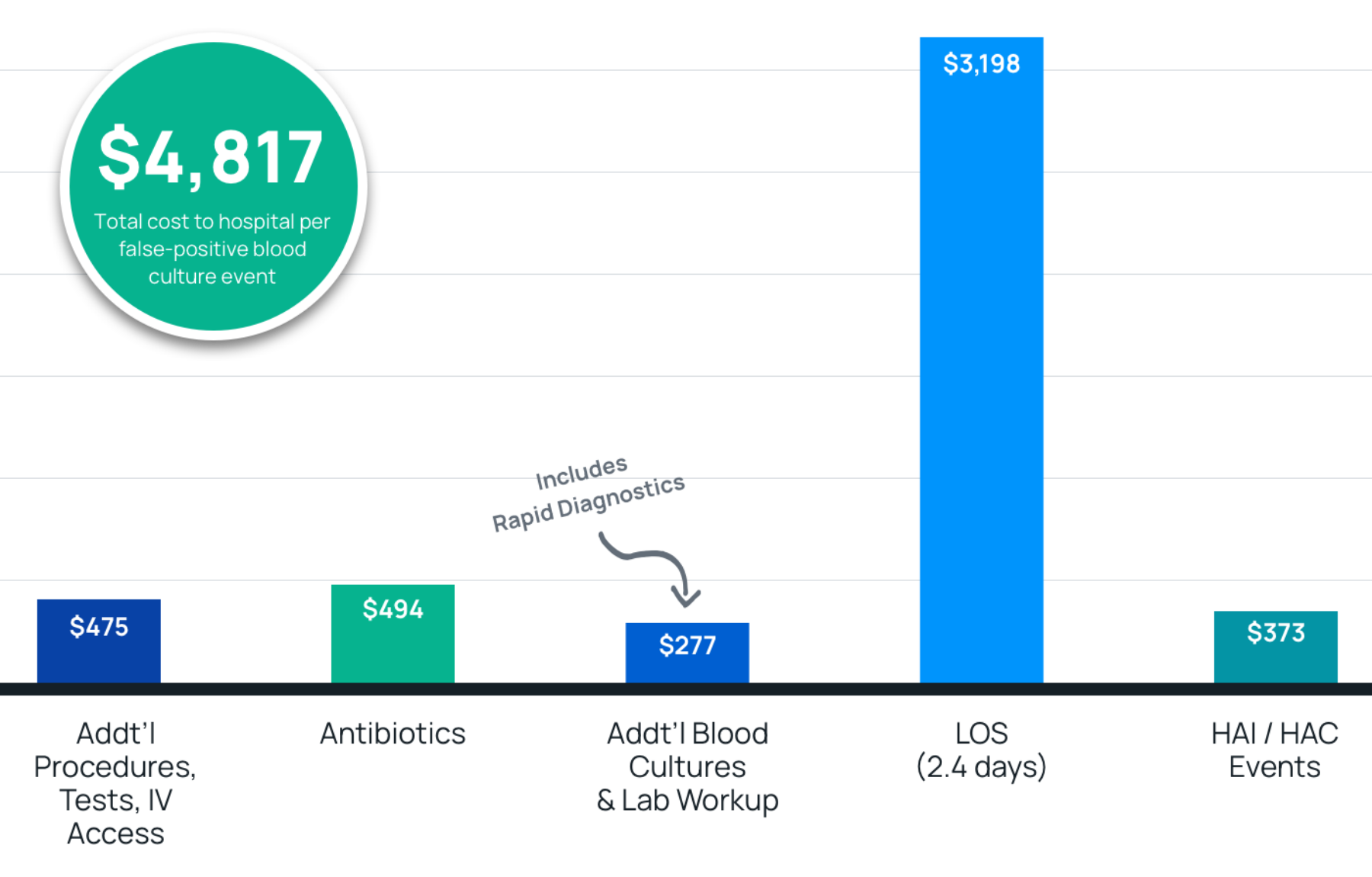
- Results: $4,817 in total costs and a length of stay increase of 2.4 days per contamination event. Estimated costs savings were $186 per blood culture, after adoption of Steripath®.
- Summary: The use of Steripath (ISDD®) is the single most effective intervention explored so far for reducing costs related to false-positive blood cultures, saving the typical 250-400 bed hospital $1.9M ($186/per culture), and preventing 34 HACs (including three C. difficile cases).

We’re on a mission to improve the diagnostic accuracy and timeliness of sepsis test results.