Prevent Sepsis Misdiagnosis with Steripath®
Steripath is FDA 510(k)-cleared with a specific indication to reduce blood culture contamination4 for sepsis testing accuracy.
The Steripath Difference
Optimally designed for blood culture contamination prevention.
Steripath comes pre-assembled and sterile to actively divert and sequester the initial 1.5-2.0mL of blood, the volume known to contain contaminants.6 Blood cultures are collected through an independent, second flow path, creating a closed vein-to-bottle collection system designed to prevent bypassing diversion.
An Engineered Solution for Contamination Prevention
Training and education have long been the standard intervention, but they only show modest, unsustainable reductions in contamination rates because they cannot address potential contaminants that are viable in the keratin layer of the skin even after skin prep. Manual diversion methods to remove contaminants prior to specimen collection are impractical for busy departments and difficult to sustain and reproduce results. Passive diversion options create the potential for the contaminated blood to mix with the culture specimen, are susceptible to bypassing diversion, and do not conform to published guidelines on clinically proven diversion volumes.
Steripath prevents contaminants from entering the blood culture bottle by utilizing engineered compliance that will not allow specimen collection until diversion is complete, providing a sustained contamination rate as low as 0.0%.8
Blood Culture Results You Can Trust
Steripath’s proprietary technology and easy-to-use design help you effectively reduce blood culture contamination and false-positive test results so you can prevent patient harm, and reduce unnecessary and prolonged antibiotic usage, length of stay, and hospital costs.
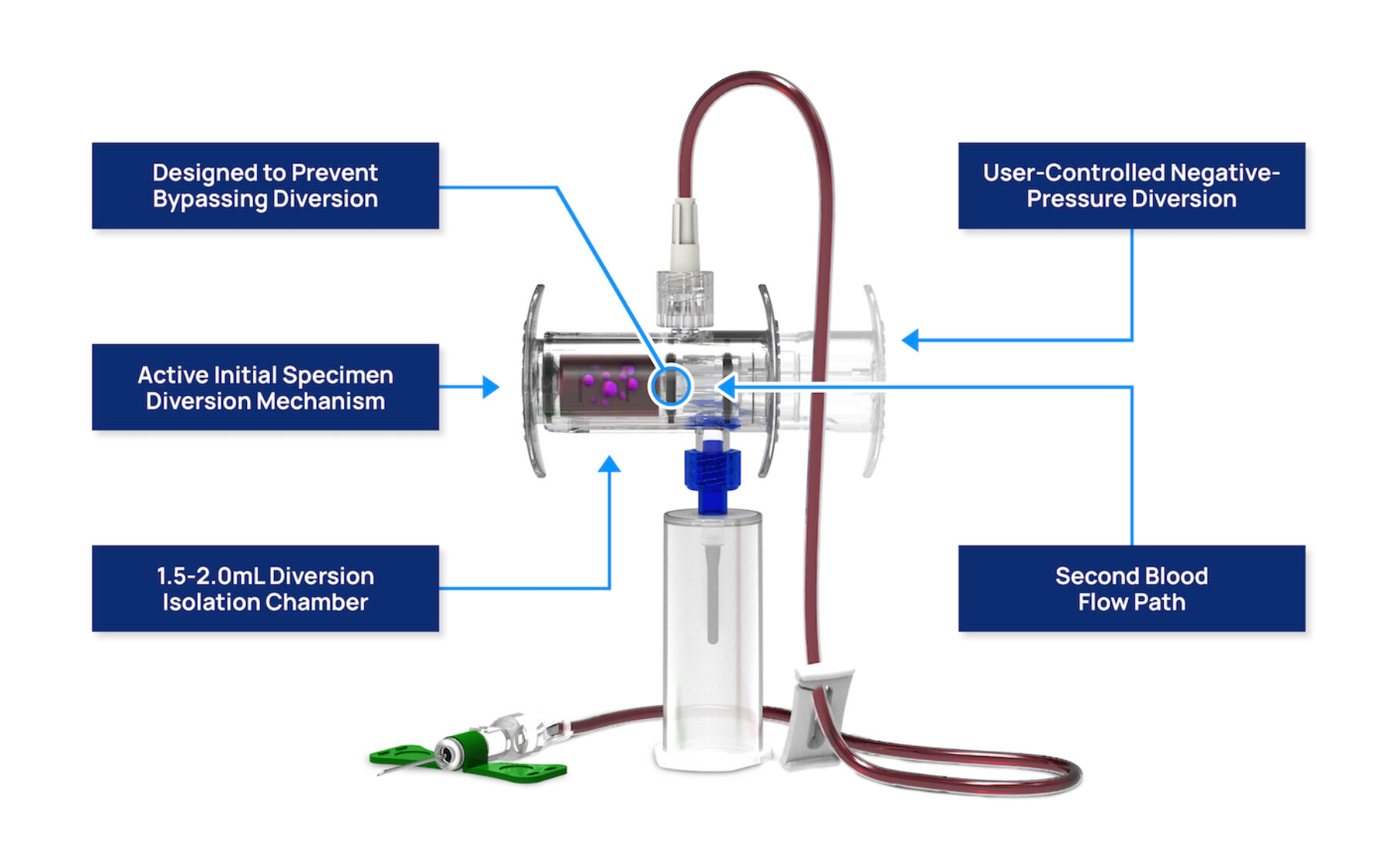
- Pre-assembled and Sterile
- Active Initial Specimen Diversion Mechanism
- 1.5-2.0mL Diversion Isolation Chamber
- Integrated Syringe Collection
- Vein-to-Bottle, Closed-System Technology
- User-Controlled, Negative-Pressure Diversion
- Second, Independent Blood Flow Path
The Steripath® device is offered exclusively with BD Vacutainer® UltraTouch™ Push-Button Blood Collection Set to provide additional benefits
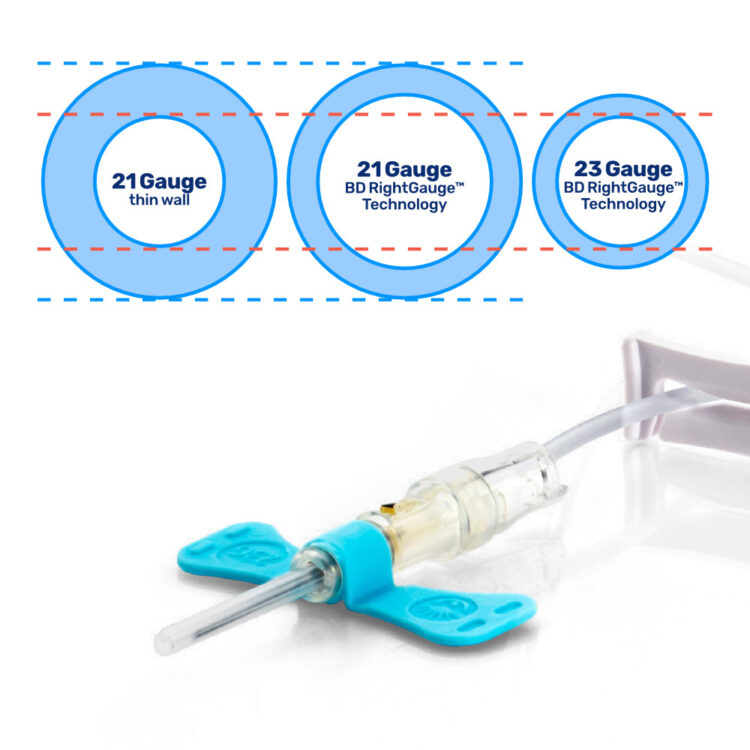
Enables the use of a smaller needle in all patients for blood culture collection.
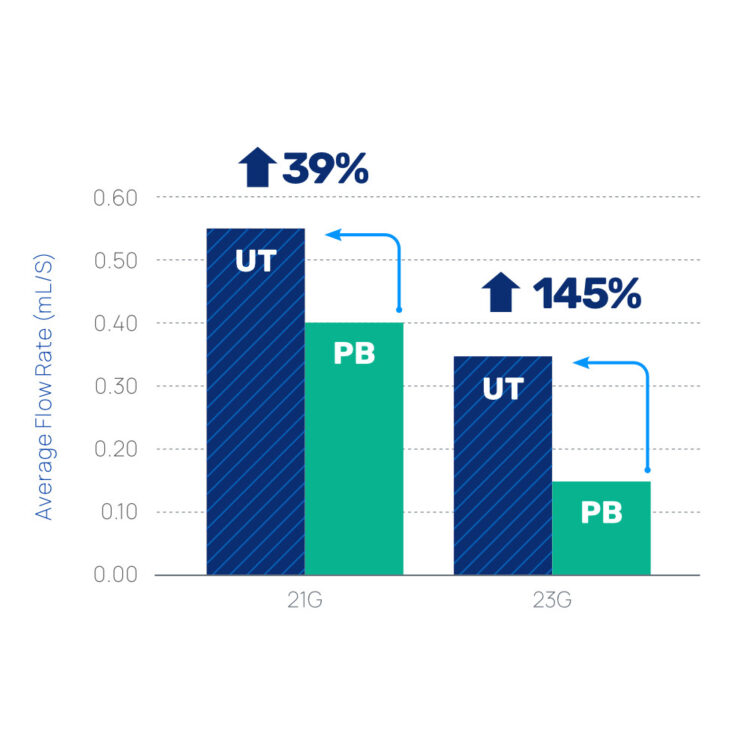
Improves sample flow rates, which helps achieve greater sample fill volume.
Report, Flow Rate Characterization, UltraTouch (UT) vs Non-UT Needles. D00672 Rev A. Magnolia Medical Technologies, Inc. 2023. Bench test results may not necessarily be indicative of clinical performance.
Seamless Integration into Your Blood Culture Protocol
Integrated:
- Transfer adapter direct-to-media configurations
- 10mL and 20mL integrated syringe configurations
Compatible with:
- BD BACTEC™ and bioMérieux BACT/ALERT™
Available with:
- 21G BD Vacutainer® UltraTouch push button blood collection set, 23G BD Vacutainer® Ultra Touch™ push button blood collection set, and CT-compatible Luer (for IV starts) extension sets
Clinical Performance Guarantee
Every Steripath device is backed by our Clinical Performance Guarantee. Reduce blood culture contamination rates at your hospital by at least 50%, or get your money back.
(Additional terms and conditions may apply)
Ready to Make an Impact?
Let us help you deliver the most consistent, accurate diagnostic results for your patients.
Steripath Ready to Make Impact
"*" indicates required fields