
We Believe the Only Acceptable Number for Sepsis Misdiagnosis is Zero
To be Innovative and Create New Standards
Magnolia Medical is on a mission to deliver breakthrough solutions that advance diagnostic accuracy to support better clinical decisions and improve patient outcomes. The impact we can have on people’s lives is what fuels our passion and gives us our purpose.
Company
News
The Cost of Blood Culture Contamination
Blood culture contamination and false-positive results for sepsis routinely lead to unnecessary and prolonged use of broad-spectrum antibiotics, which can have harmful clinical and cost consequences. 2
Patient Harm
3 million antibiotic-resistant and Clostridioides difficile Infections (CDIs) each year and 48,000 people die.
1 in 5 patients experience adverse drug event (ADE) associated with antibiotic administration in acute care hospital setting.
Wasted Resources
$6 billion is spent by our U.S. healthcare system each year on unnecessary treatment associated with false-positive blood culture results. 49
This does not include the impact blood culture contamination can have on CMS key quality outcome metrics and hospital reimbursement.
Developed by Physicians. Backed by Science. Proven Results.
The Steripath® Initial Specimen Diversion Device® is FDA 510(k)-cleared with a specific indication to reduce blood culture contamination3 for sepsis testing accuracy. The submission included clinical studies reporting an 83% and 88% contamination rate reduction for blood cultures collected via peripheral venipuncture and peripheral IV start and peripheral venipuncture only.
Steripath is the simple, all-in-one, evidence-backed solution that gives you the proven results you need for diagnostic stewardship to provide your patients with the best quality care.
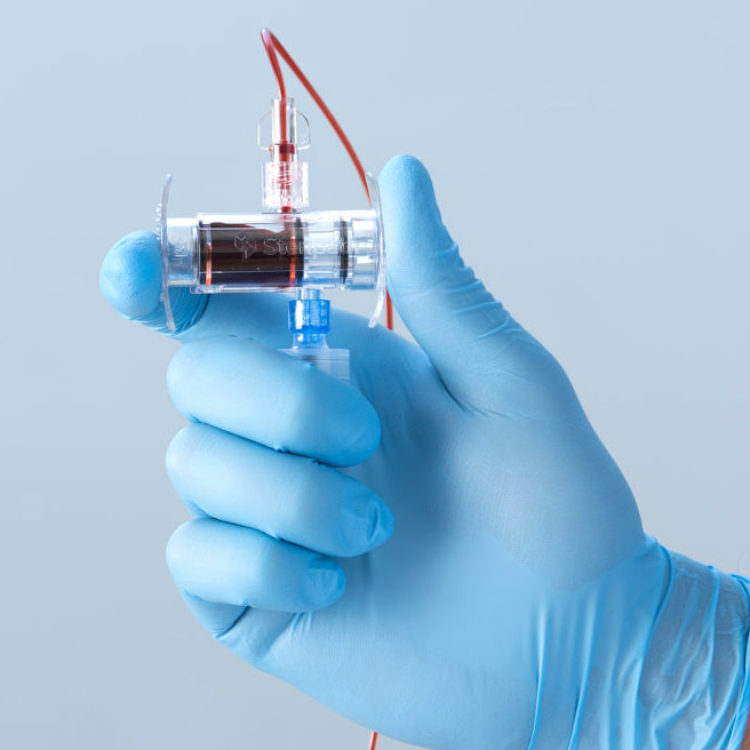
Steripath
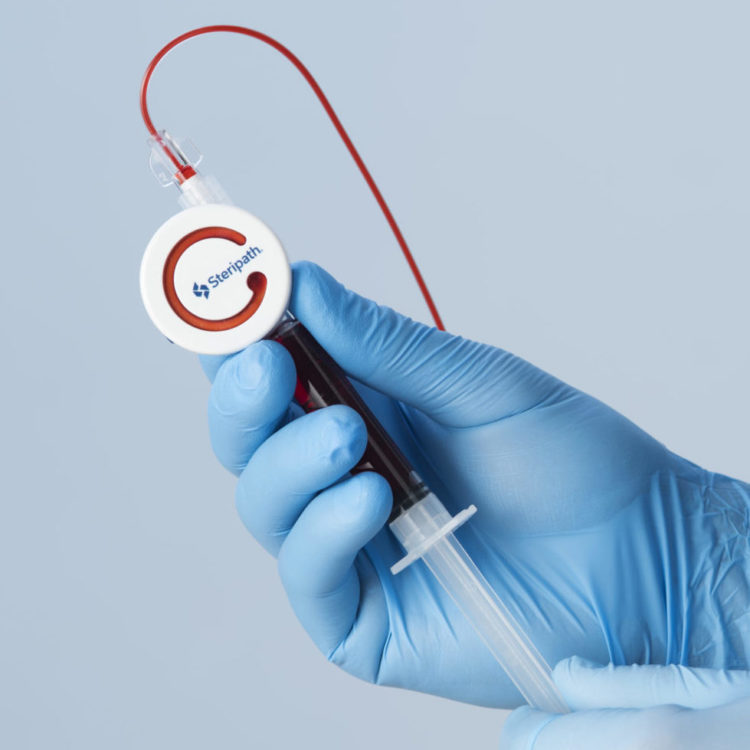
Steripath Micro
Trusted by Hospitals & Healthcare Systems Nationwide

Clinical Infectious Diseases
Reduction in blood culture contamination through use of Initial Specimen Diversion Device

Journal for Emergency Nursing
Effectiveness of a novel specimen collection system in reducing blood culture contamination rates

Journal of Hospital Infection
Initial Specimen Diversion Device® reduces blood culture contamination and vancomycin use in academic medical center

American Journal of Medical Quality
Initial Specimen Diversion Device® utilization mitigates blood culture contamination across regional community hospital and acute care facility








We’re on a mission to improve the diagnostic accuracy and timeliness of sepsis test results.