Sepsis Testing Accuracy Technology Enables Enhanced Patient Safety, Experience and Satisfaction
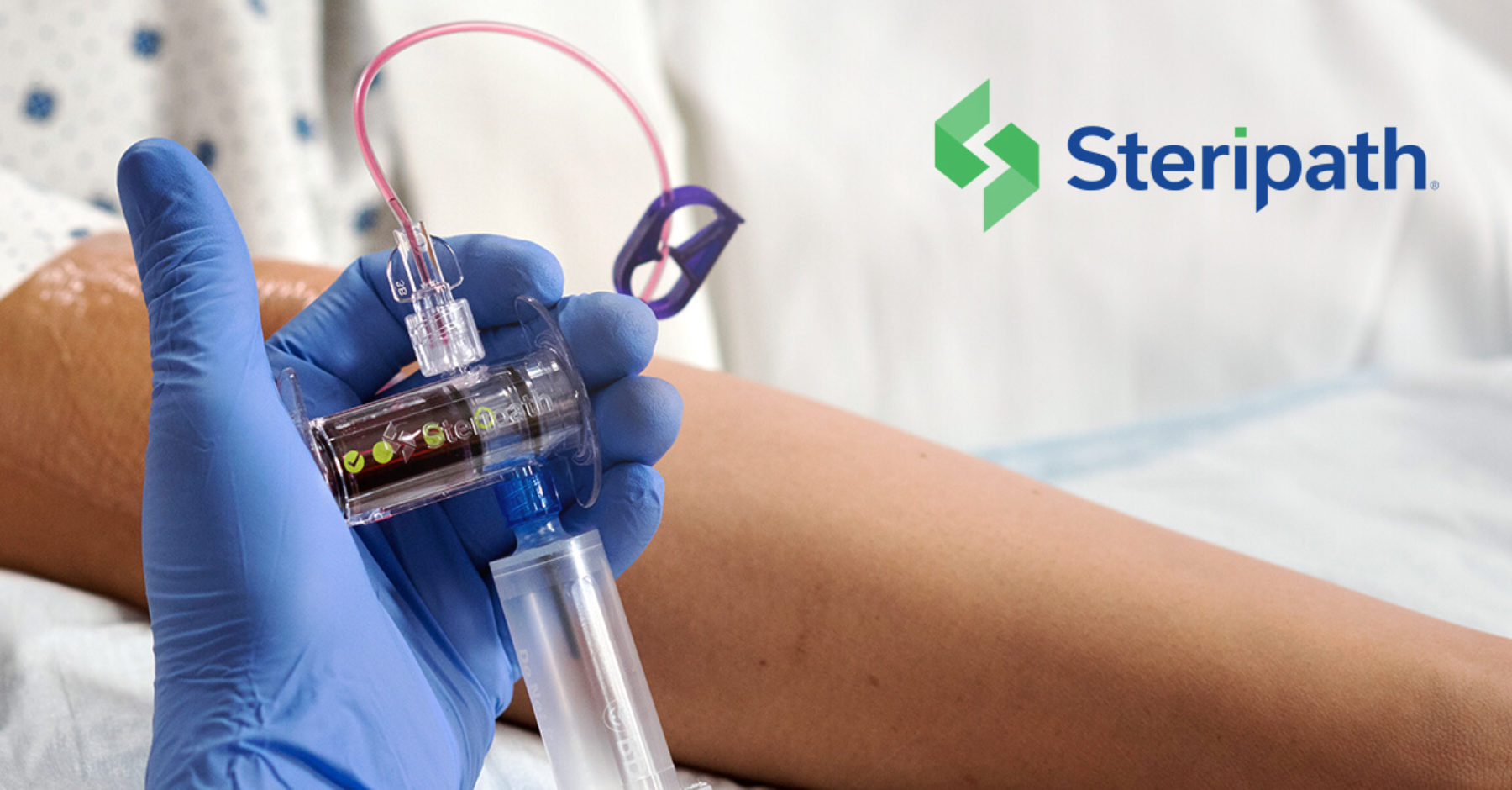
Steripath can eliminate unnecessary venipunctures by enabling blood culture draws from peripheral IV starts
SEATTLE, WA, June 9, 2021 – / PRNewswire / – Magnolia Medical Technologies, Inc., inventors of Steripath®, the only FDA 510(k)-cleared device platform indicated to reduce blood culture contamination,1 announced today continued clinical efficacy data supporting multiple, peer-reviewed clinical studies that confirm zero or near-zero false-positive rates from blood cultures drawn from peripheral intravenous (PIV) starts. These data signify that patient harm from avoidable venipunctures and sepsis misdiagnosis can be prevented.
Sepsis is a complex and aggressive syndrome impacting at least 1.7 million people in the U.S. annually of which nearly 270,000 die.2 When a patient presents with sepsis symptoms, clinical protocol often dictates that among other interventions, two PIVs are inserted for venous access to facilitate rapid treatment and two blood cultures are drawn to rule out or confirm diagnosis. These hospital and laboratory protocols typically disallow blood culture draws from PIVs because microorganisms on the skin and IV hub can contaminate the test blood specimen. Consequently, patients must endure an additional 1 to 2 needle sticks.
The Steripath Initial Specimen Diversion Device® (ISDD®) diverts and sequesters the guideline-recommended blood volume most likely to contain skin-residing microorganisms in an easy-to-use sterile, vein-to-bottle closed system. By engineering human factors out, Steripath has been proven to deliver blood culture contamination rates approaching zero via both venipuncture and PIV starts.
“By reducing the number of venipunctures, Steripath enables us to improve diagnostic stewardship while doing what is best for patients and our nurses,” said Mary Bell, MS, RN, CEN and principal investigator of a large, multicenter study published in the Journal of Emergency Nursing. “Our study demonstrated that even when the majority of 6,293 cultures were drawn from PIV starts, blood culture contamination rates were only 0.6%.3 Today, over 85% of blood cultures across four Lee Health emergency departments are drawn from PIV starts using Steripath.”
“Training, education and best practices can incrementally impact blood culture contamination rates,” said Greg Bullington, CEO of Magnolia Medical, who cofounded the company with Dr. Richard Patton – former Chief of Pathology and Medical Director of Clinical Laboratories at University of Washington Medicine, Northwest Medical Center.
“But published studies and real-world evidence show that Steripath, along with best practices, can consistently deliver sustained zero or near-zero contamination rates with blood culture draws via both venipuncture and PIV starts. This in turn expands clinical practice options, improves patient and clinical staff experience plus builds trust in the accuracy of the diagnosis,” said Bullington.
Magnolia Medical Technologies develops, manufactures, and markets innovative blood and bodily fluid collection devices to facilitate significant improvements in the accuracy, consistency and predictability of critical laboratory tests. Magnolia Medical invented and patented the Initial Specimen Diversion Technique (ISDT™) and Device (ISDD®) for blood culture collection and contamination prevention. The company has amassed an intellectual property portfolio including more than 70 issued method, apparatus and design patents with more than 50 additional patent applications pending. For more information, visit www.magnolia-medical.com.
References: 1. Indicated for use as a blood collection system that diverts and sequesters the initial specimen prior to collection of a subsequent test sample to reduce the frequency of blood culture contamination when contaminants are present in the initial blood sample compared to blood cultures drawn with standard procedure without manual diversion. 2. M. Bell, et al. Journal of Emergency Nursing (2018). 3. M. Rupp, et al. Clinical Infectious Diseases (2017)